Congenica Oncology
The time has come to change the way we treat cancer
Over a quarter of all individuals diagnosed with advanced cancer are now eligible for a treatment based on the genomic analysis of their tumour, driven by the ever-increasing availability of biomarkers and associated treatments.
However, integrating molecular tumour profiling into routine clinical care remains challenging, and the diagnostic journey for laboratories, patients and their treating clinicians is still complicated, expensive and slow.
Analysis and interpretation of the often large and complex data sets has been identified as the biggest bottleneck, together with the inability to provide accurate and relevant therapy recommendations in user-friendly reports that enable rapid patient treatment.
Transforming Cancer Care
Watch our Satellite Symposium from ESHG 2023 to hear:
-
Andrew Biankin talking about his experience in transforming cancer care by use of genomic testing
-
Philip Beer discussing new approaches to automate somatic driver annotation for evidence-based variant interpretation and increased patient sample throughput
-
Gavin Xu introducing Congenica’s novel Precision Oncology solution.
Automation is key to accelerate the diagnostic journey and drive Precision Oncology
At Congenica we have developed a fully automated CE-IVD approved Precision Oncology platform for rapid and unbiased interpretation of next generation sequencing data without the need for manual intervention. This not only reduces reporting times from hours to minutes, but eliminates the need for difficult-to-hire expert staff and paves the way for cost-effective routine cancer care.
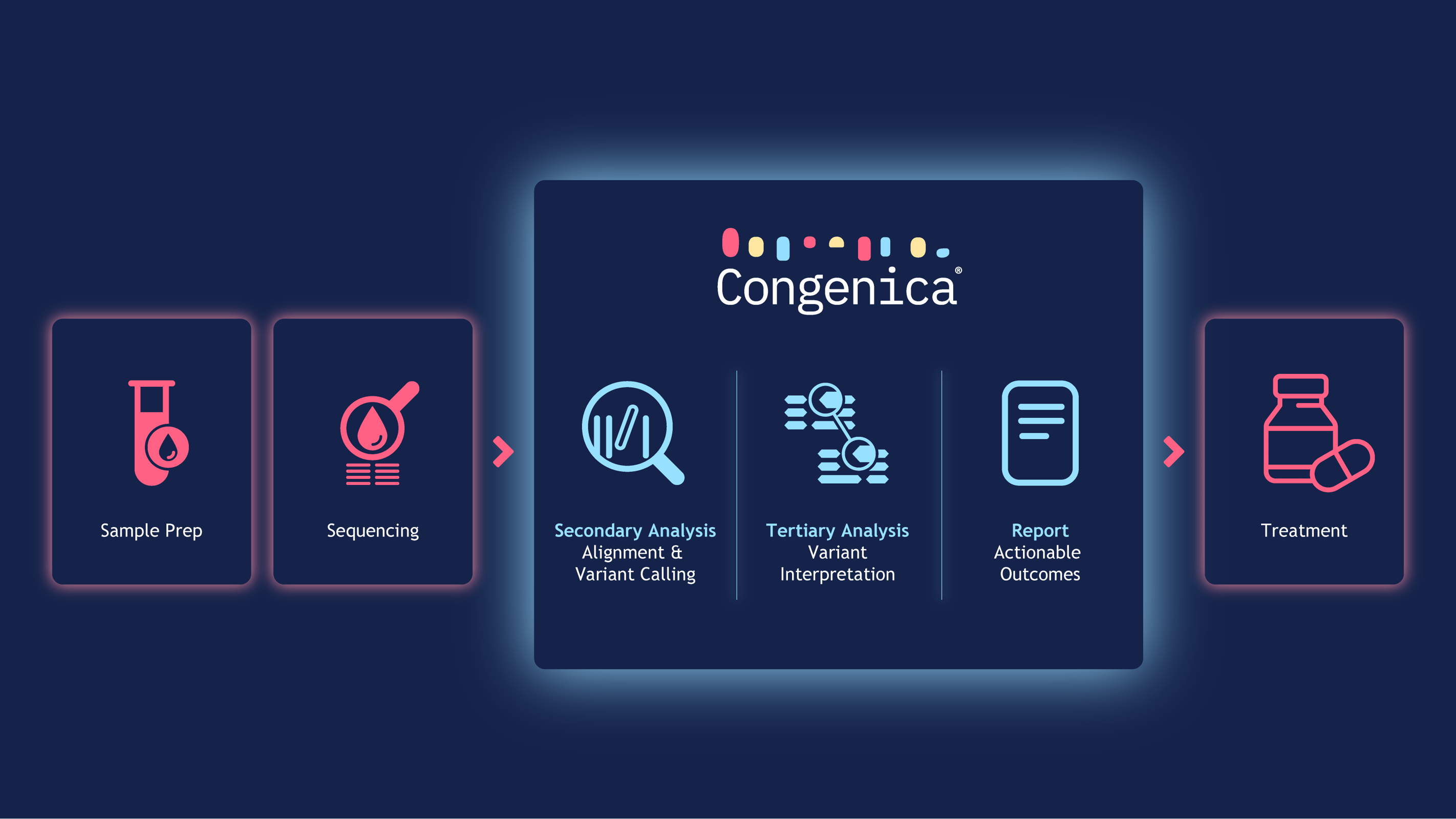
From data to report without manual intervention
Our future-proof end-to-end solution consists of fully automated and integrated secondary and teritary analysis pipelines coupled with automated, user-friendly reporting that is focused on actionable insights to support rapid treatment decisions. Automated treatment matching is powered by NCCN, ESMO, NICE and SMC best practice guidelines with authorised therapeutic assertations from the FDA, EMA and MHRA.
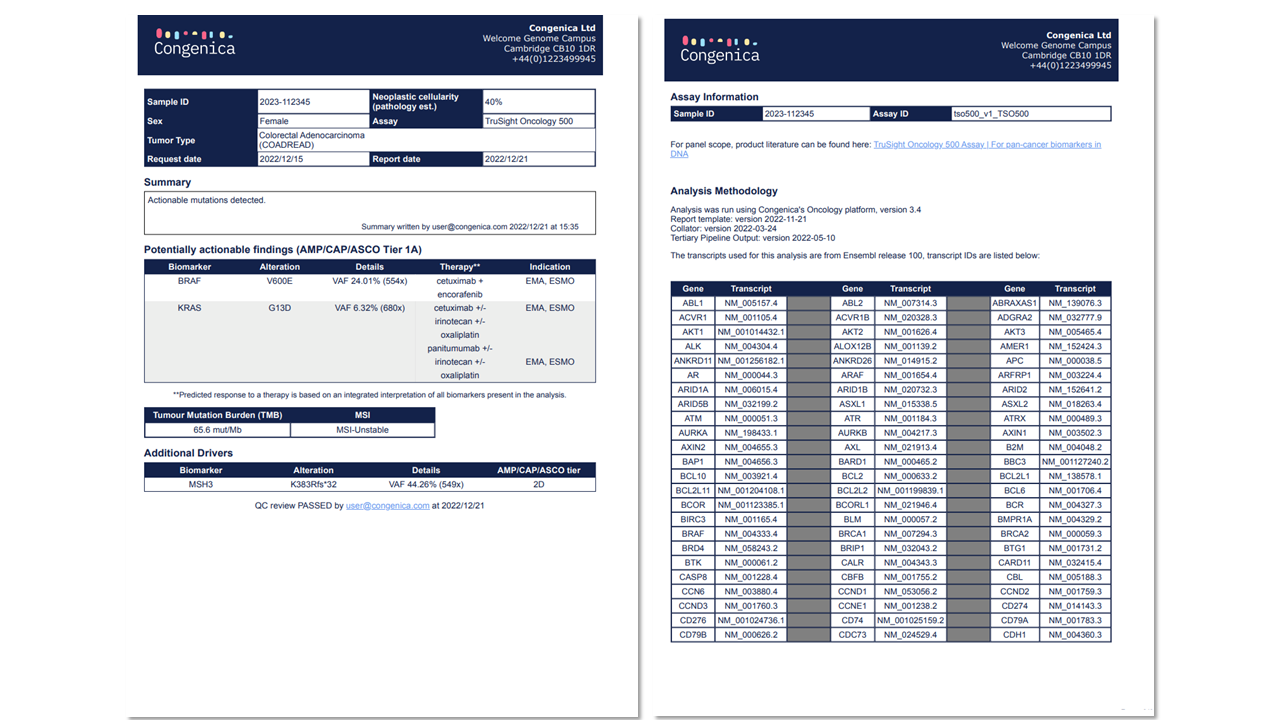
Panel support
Our highly flexible platform will initially support Trusight Oncology 500 assays, but please contact customer support if you would like us to integrate other commercial, custom or exon panels into the software.
Cost-effective
Fully automated, scalable end-to-end solution without the need of manual intervention
Unbiased
Evidence-based provision of actionable insights based on AMP/ASCO/CAP guidelines
Actionable
Rapid therapeutic matching based on regional best-practice guidelines
CE-IVD certified
Validated, accurate and secure platform for high confidence in diagnostic outcome
%20(2).png?width=1920&height=1080&name=Untitled%20(1920%20%C3%97%201080%20px)%20(2).png)



